Table of Contents
Dissolved gases in water
In addition to other contaminants, natural water or raw water that is directly sourced from resources has various gases dissolved in it. These gases are produced by atmospheric air. In terms of boiler feed water, we are primarily concerned with oxygen and carbon dioxide, but other gases still make up a modest portion. These gases have an effect on the lifespan and operation of the boiler. Oxygen causes corrosion, which eventually causes the system to fail. It also lowers the quality of the steam, uses more fuel, reduces efficiency, and causes steam leakage. which is undesirable for a boiler operation that runs smoothly.
Below is a detailed description of how oxygen and carbon dioxide affect boiler performance.
Effect of oxygen
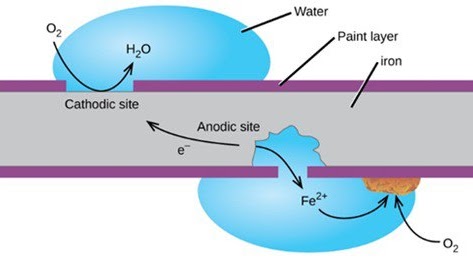
One of the most dangerous gases in water is oxygen; even a relatively small amount can seriously destroy the boiler system. It causes rust and corrosion known as oxygen pitting or oxygen attack when it reacts with iron-carbon steel. Oxygen is more corrosive at higher temperatures. This oxygen attack may be localized or contained to a specific area, which ultimately results in system failure or leakage.
Pits are small, localized corrosion spaces created by oxygen. Oxygen pits may quickly “drill” through metal surfaces, which causes metal to become worn down and fail. The iron surface dissolves as oxygen corrodes the boiler metal. In addition to weakening the metal site, this also introduces dissolved iron into the boiler. This dispersed iron can build up on boiler tubes, scorching them and leading to tube failure.
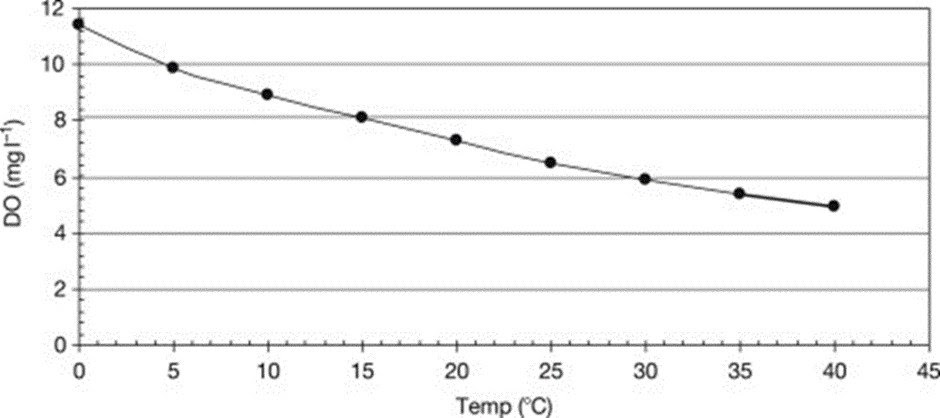
Temperature and pressure affect how soluble oxygen is in water; at constant pressure, solubility reduces as temperature rises, whereas at constant temperature, solubility rises with pressure.
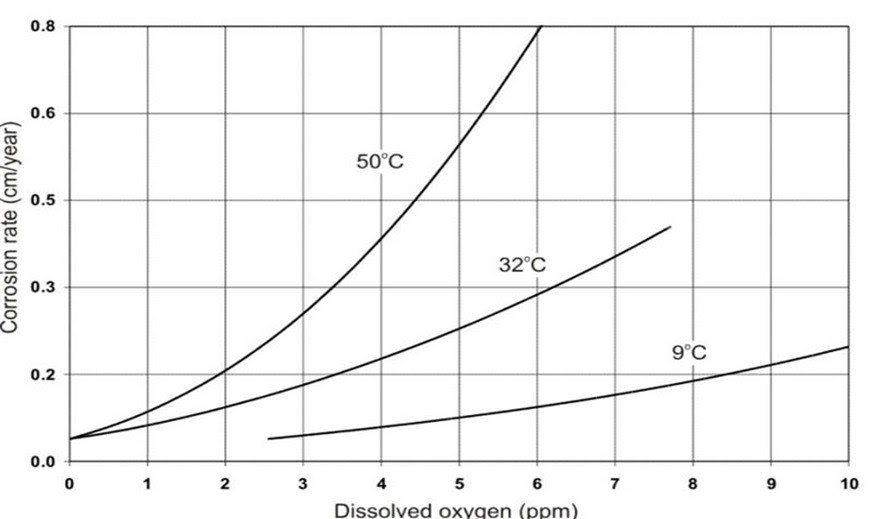
When temperatures are high, oxygen becomes more corrosive and breaks down metal more quickly than when temperatures are low. This accelerates the system’s breakdown.
Effect of Temperature on Corrosion
Effect of carbon dioxide:
Thermodyne Boilers
Because the deaeration process removes the gases from the feed water, the make-up water after deaeration often has no CO2 content. It is the condensate that contains CO2 most frequently, therefore the condensate tank quite often experiences CO2 corrosion. The decomposition of carbonate and bicarbonate in condensate under boiler conditions is typically what causes the presence of the gas in condensate. Both oxygen and free carbon dioxide can be removed from the feed water by heating it in the feed tank and keeping it there while it is being delivered to the boiler.
Dissolved Gases in Feed Water and Its Effect FAQ
1. What are dissolved gases?
Dissolved gases are gases that are present in water. They can come from the air, from the ground, or from other sources. The most common dissolved gases are oxygen, nitrogen, carbon dioxide, and hydrogen sulfide.
2. What are the effects of dissolved gases in water?
The effects of dissolved gases in water can be both positive and negative. Positive effects include:
Oxygen is essential for aquatic life.
Carbon dioxide helps to regulate the pH of water.
Nitrogen helps to prevent corrosion.
Negative effects include:
Hydrogen sulfide can be toxic to humans and animals.
High levels of dissolved gases can cause water to become corrosive.
Dissolved gases can interfere with the treatment of water for drinking and other purposes.
3. Why should the presence of CO2 in boiler feed water be avoided?
Carbon dioxide (CO2) can react with water to form carbonic acid (H2CO3), which can cause corrosion of boiler tubes. CO2 can also interfere with the efficiency of the boiler by reducing the heat transfer rate.
4. What are the ways to remove dissolved gases from water?
There are a number of ways to remove dissolved gases from water. Some common methods include:
Aeration: This involves exposing the water to air, which allows the gases to escape.
Vacuum deaeration: This involves using a vacuum to remove the gases from the water.
Chemical deaeration: This involves adding chemicals to the water that react with the gases and remove them from the solution.
5. What are the effects of dissolved gases on the taste and smell of water?
Dissolved gases can affect the taste and smell of water. For example, high levels of dissolved carbon dioxide can give water a sour taste. Hydrogen sulfide can give water a rotten egg smell.
6. What are the effects of dissolved gases on the health of humans and animals?
Some dissolved gases, such as hydrogen sulfide, can be toxic to humans and animals. High levels of dissolved gases can also cause respiratory problems
7. What are the effects of dissolved gases on the environment?
Dissolved gases can have a number of effects on the environment. For example, high levels of dissolved carbon dioxide can contribute to climate change.
8. What are the regulations for dissolved gases in water?
The regulations for dissolved gases in water vary from country to country. In the United States, the Environmental Protection Agency (EPA) sets standards for dissolved gases in drinking water.
9.What are the best practices for managing dissolved gases in water?
The best practices for managing dissolved gases in water vary depending on the specific application. However, some general best practices include:
Regularly monitoring the levels of dissolved gases in water.
Using methods to remove dissolved gases from water as needed.
Ensuring that water is treated to remove any harmful dissolved gases before it is used for drinking, cooking, or other purposes.
10.What are the future trends for dissolved gases in wate
The future trends for dissolved gases in water are likely to focus on:
Developing new methods to remove dissolved gases from water more efficiently and cost-effectively.
Developing new technologies to use dissolved gases for beneficial purposes, such as generating electricity or producing fuels.